For centuries, copper has proved to be one of the best, most cost-effective architectural metals for corrosion resistance. Along with stainless steel and titanium, it often is used where corrosion is a major concern. Although no roofing material is perfect, copper can satisfy the demands of building owners, architects and contractors when it comes to lifetime economy, ease of fabrication, low maintenance and environmental friendliness.
There are thousands of examples of long-lived copper roof system and flashing installations, many well over a century old. Time has proved those buildings were constructed properly with careful thought to design, details and installation. But many factors can limit the potential life of metal roofing and flashings, one of which is corrosion.
Atmospheric corrosion
The surface of any architectural metal will react when exposed to certain chemicals. Some chemicals are natural, such as those found in water from rainfall. Others are manmade, such as industrial pollution. How a given metal reacts to a chemical is related to the type of chemical, chemical's concentration and amount of time to which the metal is exposed to the chemical.
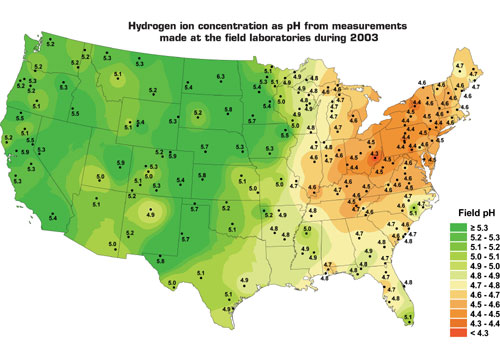
Figure courtesy of the National Atmospheric Deposition Program, Champaign, Ill.
Figure 1: The progressively darker red areas on the map indicate the lowest pH levels and areas most prone to problems from acid rain.
Corrosion—the deterioration or wearing away of metal—can be a reaction to a chemical. A given concentration of a chemical may cause moderate corrosion on one metal, extreme corrosion on a second and have virtually no effect on a third. Some metals corrode relatively rapidly and require either protective coatings (such as paint) or sacrificial coatings (such as the zinc layer that covers galvanized steel).
Oxidation—when a metal surface combines with oxygen and forms a coating—is another reaction. Unlike oxidation on most steels (commonly know as rust), copper oxidation is good. It provides a film, or patina, that protects copper from corrosion. For example, The Statue of Liberty has developed a lovely blue-green patina that has protected the statue's copper skin from corrosion since 1886. Measurements show the monument has lost only about 0.005 inches (0.127 mm) of its surface in all that time.
Copper naturally reacts to its environment and goes through a weathering progression, the rate of which is related to its degree of exposure to moisture, salt and atmospheric pollutants such as sulfur. In some arid climates, it will mature to a nut-brown or ebony-like patina. In coastal or moist areas, the patina will progress to variations of a grayish-bluish-green. Usually, within 10 years to 30 years, copper will reach a weathering equilibrium where the oxide film stabilizes and no longer changes color; however, it continues to protect copper from aggressive corrosion attack.
Acid rain
Under some extreme conditions, copper (and other metals) may corrode before a patina or protective oxide film can form. Such an occurrence usually is associated with acid rain.
Acids are chemicals that detrimentally may affect metals. The term "pH" is used to express a solution's acidity or alkalinity on a logarithmic scale of 0 to 14 where less than 7 represents acidity, 7 neutrality and more than 7 alkalinity.
Precipitation (rainfall, snow, dew, fog) typically is acidic, primarily because of the reaction of water with naturally occurring carbon dioxide. Rainfall from unpolluted air usually has a pH of about 5.6. Precipitation containing relatively high concentrations (lower than 5.5 pH) of acid-forming chemicals is called acid rain. It derives from pollutants created from the burning of fossil fuels, chemical manufacturing processes, and other procedures that release sulfur and nitrogen oxides into the atmosphere and combine with water vapor.
Acid rain is nothing new—we've known about it for more than 150 years. But it has become a serious problem since the 1920s. In recent times, most areas of the world have been taking significant steps to stem its levels.
Unfortunately, acid rain persists and can cause problems. For example, over time, the cumulative effects of acid rain can cause corrosion of architectural metals, including copper. The overall effects of acid rain directly are related to the pH of the moisture, the length of time acidic moisture is in contact with the metal (known as "dwell time") and sheltering (blocking of the sun, which can prolong moisture dwell time).
Who's at risk?
The National Atmospheric Deposition Program (NADP), Champaign, Ill., publishes annual pH pictures of the continental United States on its Web site, nadp.sws.uiuc.edu. (See Figure 1.) A comparison of this map with one a decade earlier shows no significant difference in pH levels. It appears efforts to reduce acid rain only have succeeded in checking its increase.
NADP data help provide a general alert to where there may be potential corrosion problems; however, building designers and installation contractors also should evaluate the local environmental conditions to which a structure will be exposed. For a given area, observation of existing local structures can provide clues to the presence of harmful acid levels. Look for excessively shiny metal surfaces compared with surrounding areas and pitting or ragged edges next to an obvious loss of material (see Photo 1). The presence of pitting or flaking is a key determinant of corrosion, but laboratory analysis may be necessary. Black or discolored copper typically is a result of natural weathering or chemical reactions with other materials and is not necessarily a sign of corrosion.
Architects and contractors who work with sheet metal know accelerated corrosion can occur on any metal if used improperly. In potentially problematic areas, it's a good idea to use the most corrosion-resistant architectural metals possible, such as copper, stainless steel and titanium.
Using an alternative material with an expected lifespan of 20 years or 30 years for an entire roof to protect against the potential of accelerated corrosion in a few areas may not be appropriate when determining life-cycle costs for a roof system built to last 100 years.
If a roofing material has an estimated lifespan of 25 years, and copper has an estimated lifespan of 100 years or more, it is a comparison of the cost of one copper roof system installation with the cost of four alternative-material installations. In constant dollars, that would be approximately 4 to 1. It's much more practical to take some special care to prevent the few potential problem areas you might encounter.
Using copper
Keep in mind active corrosion only can occur when a metal's surface is wet. And the only time acid rain causes a problem is when it is allowed to remain in contact with a metal. So regardless of a structure's geographic location, use conservative recommendations and details, such as those found in the Copper Development Association's (CDA's) Copper in Architecture Handbook. (Editor's note: Details also can be found in NRCA's Architectural Sheet Metal and Metal Roofing Manual.) Proper watershedding design and detailing can prevent a vast majority of atmospheric corrosion problems by reducing the dwell time of acidic water on metal surfaces.
For years, industry professionals have recognized the potential for corrosion when acidic water is concentrated on a small area of copper or other metals. For example, this may occur when rain falls on a large area of some roofing materials such as tile, slate, wood or asphalt and then flows downward. The acidic water may not be neutralized as it flows over those materials. But when that water is diverted or collected by a relatively small copper flashing or gutter, the copper may deteriorate before it develops a protective patina.
Conversely, concentrated runoff from limestone or granite usually will not cause a problem because these materials tend to neutralize water's acidity.
Another type of corrosion occurs at the drip edge of a relatively inert roofing material that conducts water into a copper gutter or valley.
If the up-slope material—often shingles—rests directly on metal flashing, the corrosive effect is amplified because moisture is held along the edge by capillary action. The result is "line corrosion."
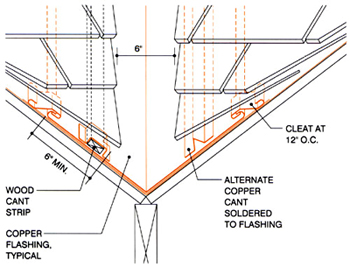
Detail courtesy of the Copper Development Association
Figure 2: In this detail, the cant strip raises shingles, avoiding edge contact with the flashing surface. This precludes capillary action that would retain water and could lead to line corrosion. Roof intersections should result in valley slopes of at least 4 1/2 inches per foot.
As shown in Figure 2, the solution is to provide a cant strip between the shingles and copper to provide a capillary break. (Editor's note: CDA considers a cant strip in this case to mean "a wood board or a formed copper strip [that] is laid so as to cant the first row of shingles on a roof." NRCA defines a cant strip as "a beveled strip used under flashings to modify the angle at the point where the roofing or waterproofing membrane meets any vertical element.") A similar approach can be taken with flashings where there are changes in roof slopes. (See Figure 3.)
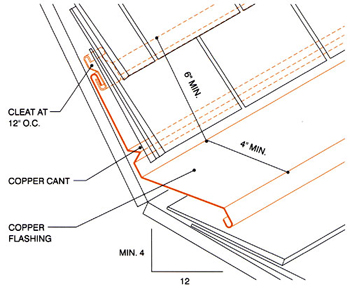
Detail courtesy of the Copper Development Association
Figure 3: This is a flashing detail at a change-of-slope location. If slate or tile is used for the roof covering material, a minimum 20-ounce plain or lead-coated copper is recommended for the valley flashing; otherwise, 16-ounce copper may be used.
Still another solution is appropriate where snow may remain on the roof for extended periods and there's a potential for ice dams (see Figure 4).
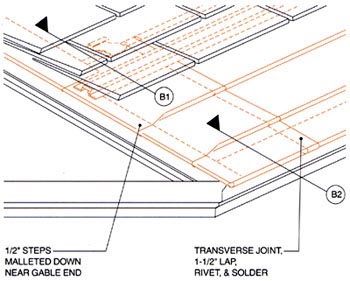
Detail courtesy of the Copper Development Association
Figure 4: When melted snow reaches exposed eave overhangs, it can freeze and form ice dams, causing water to go under the roofing material. This snow flashing detail helps prevent leaks and emulates a shingle's butt line, and the copper cant strip protects against line corrosion.
Slope recommendations
Most sheet-metal joinery is designed to be watershedding. CDA recommends slope requirements for copper roof panel systems and flashings to allow water to rapidly flow from a structure so it won't infiltrate joints or pond. When ponded water evaporates, the water's acid concentration increases and can reach the point where it adversely will affect the metal, resulting in corrosion.
The solution is simply to avoid designs that don't allow sheet metal to shed or drain water. Even the top of a coping needs slope. The wider the coping and lower the slope, the greater the risk of ponded acidic water. (See Figure 5.)
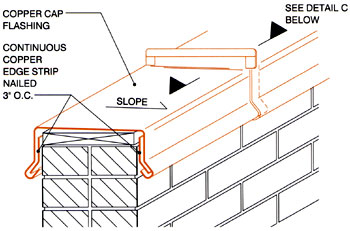
Detail courtesy of the Copper Development Association
Figure 5: Horizontal top surfaces of walls are the most vulnerable point for water to enter and, unless their copings have slope, are susceptible to the effects of acidic standing water.
Gutters should slope to drain and be able to accommodate anticipated runoff. The amount of runoff is related to roof area, slope and local rainfall. Use thicker copper in potential problem areas.
Even metal cladding systems designed to be watertight, such as soldered flat-lock roofing, must incorporate slope and encourage water to drain.
It is important details and shapes be formed properly to shed, not trap, moisture. Copper is more malleable than other architectural metals and facilitates even the most complicated shapes. Just be sure the shape itself does not present a potential problem.
The devil in the details
Unfortunately, poor details may be presented by architects or contractors, many times under the false assumption there will be savings in time or money. In other cases, it's because of inexperience.
For example, it's not uncommon for those who usually work with prepainted steel or aluminum products to suggest details that rely on sealants. In most cases, sealants should not be necessary with a properly designed copper installation; and, where appropriate, solder should be used instead of an adhesive or sealant. Where strong, watertight joints are required, such as for internal gutters, copper systems can be joined by soldering. Other commonly used metals are either difficult to solder properly or must rely on sealants, which are, at best, a relatively short-lived solution requiring frequent maintenance.

Detail courtesy of the Copper Development Association
Figure 6: This weathering cycle represents a copper panel at 45 degrees with a southern exposure in a typical Northeastern industrial city.
All details should be reviewed carefully. Omissions or improper alternatives may save a bit of material cost and labor in the short term. But, ultimately, they will compromise the performance of an entire system by eliminating a proven method to prevent corrosion.
Repairs?
An advantage of copper is that it is relatively easy to repair. There are a few simple steps that can be taken if you encounter a problem area. Consider the following:
Although paints and sealants could be used, they are short-term solutions. Often these "quick fixes" cause more problems than they solve.
As with any architectural metal, know what you are buying and its source. Specify copper products of appropriate thickness and temper for the application. Insist architectural sheet copper is manufactured according to ASTM B370-03, "Standard Specification for Copper Sheet and Strip for Building Construction." Accepting any substitution can lead to increased liability and inadequate performance.
Cold-rolled 1/8 Hard temper (H00) copper is recommended for most roofing and flashing installations. Thickness (weight) will vary, typically between 16 ounces and 32 ounces, depending on specific application. CDA's handbook provides guidance for most situations.
Assistance
For centuries, copper, when used properly, has shown its mettle in resisting corrosion at acid-rain levels. However, if you have concerns about potential corrosion issues, need design assistance or detail reviews, or would like to learn more about using and installing architectural copper, access CDA's Web site at www.copper.org and click on "Architectural Applications."
Larry E. Peters is a regional manager of the Copper Development Association.
COMMENTS
Be the first to comment. Please log in to leave a comment.